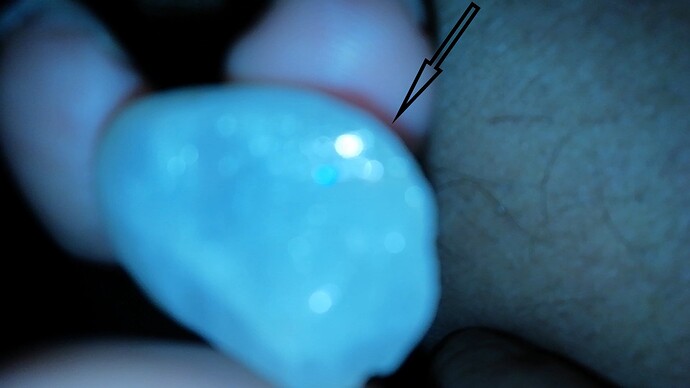
Unfortunately, I can’t offer much help, but I have two stones that look (as far as I can see in the pic)c newly the same. I had never heard of such a diamond, and if such a stone exists, I’m anxious to learn more about it. Do you happen to have more clear pics of the stone not under UV? Does it have inclusions or any kind? Any idea where is was found/mined?
The stone was taken under white light not UV and it shows blue sparkling under white light. clarity looks good to naked eye and the stone was found in Sri Lanka
Regardless of what the stone actually is, its quite beautiful.
- The body color is not typical of a blue diamond. Pale blue diamonds have a grey tone to them.
- The fracture is also wrong for a diamond. Diamond fracture patterns tend to be substantially sharper. Yours appears wavy, similar to how beryls fracture.
- The Clarity is also wrong. Your stone is better described as “milky.”
Based on the color, fracture, and milkiness, I am inclined to conclude the stone is most likely not a diamond.
Here are some better matches:
Number 1 match:. Sri-Lankan Opalite moonstone: Same body color, clarity, and fracture pattern. Also, some silicates can fluoresce that particular blue shade.
Best Runner Up: Milky aquamarine. Sri-Lanka also produces aquamarines. Aquas are renowned for that pale blue body color (fantastic example: Beryl Mineral Specimen For Sale). The fracture is a little off, but can be explained away if this stone came from an alluvial deposit. Tumbled aquamarines can be cheaply found with the same milkiness. No references that indicate a blue florescence that I am aware of.
Close 3rd: Goshenite. Also in the beryl family. Can also be found in Sri-Lanka. Some examples can fluoresce blue.
Do they find many tumble polished diamonds, and if so, how’s it done? ![]()
Diamonds are not just found like that. You will never see them come out of the ground being that rounded. If that is a diamond it would have been cut that way, your stone has not been cut. Also, looking at fracture points and chips in your stone, this doesnt contain the internal structure of a diamond. I would side with others who say that this is a beryl (aquamarine).
what about diamond tester results, It shows excellent thermal conductivity. .The probe of the tester comes up quickly once it is touched
blue or star like blue sparkle is clearly visible not light blue, you might not see it in the pic or you dee it as light blue
Beryllium containing stones, (including all members of the beryl family), have high thermal conductivities ( 200 W/(m⋅K).
All a diamond tester is really good for, is separating natural from synthetic diamonds.
Diamond testers should be considered “confirmative” not “determinative” testing.
Additionally composite silicates can also have high thermal conductivities due to water content.
have to agree with rlynch and others… hardness as you measured, not withstanding, very unlikely to be diamond…many silicate stones also overlap into the SG you measured. how did you test the hardness? Using a calibrated kit with known hardness testing tips and using enough pressure to try to make a scratch should be done…if it’s hardness 10, it should not scratch with carborundum ( moissanite)…which has a hardness of 9.3- 9.5. Thermal conductivity also is not definitive. More of a screening test than a determinative one.