Kadda,
As I stated in my initial response, it is nearly improbable to make any gemstone identification from an image alone. The pictures you initially presented are of a very large stone. (Which is a very nice specimen, by the way).
So let us dissect this conundrum from the corundum.
I understand that your initial question was to determine whether or not additional money should be spent or as suggested, use it for carving ashtrays. If it is corundum, carving ashtrays would be an arduous task, due to the typical Mohs hardness of 9.
Therefore, based on visual features of the stone you presented:
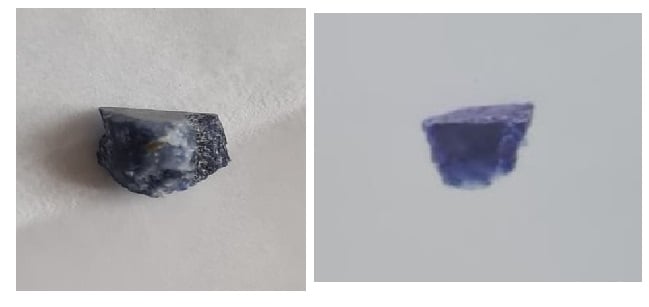
- Large patches of blue to violet areas with ultra-fine crystalline granularity.
- Large fractured inclusions of white.
- No visual presence of pyrite within the matrix as a whole.
- Vitreous and Greasy luster. (although this may not very reliable because the specimen has been worked.)
I offered an assessment from the images which is consistent with the classic coloration and large inclusions of white calcite, interspersed with some quartzite, commonly found in Sodalite. The lack of pyrite can typically discount the specimen as being Lapis Lazuli, if presented in a rough matrix, such as seen in the images.
The two links I provided, present information that using basic rockhounding techniques, one can perform simple tests to help define or discount the classification. If your stone is corundum, it will not be scratched by a steel nail or by Glass / Quartz. Sodalite is much softer with a Mohs of 5 - 6. There are other characteristics that could be assessed as well. Including: specific gravity, refractive index, and Fluorescence.
However, the LFG gemological report becomes an enigma. Based on your statements, this is directly connected to the stone you presented initially. But you have not presented an image of the stone that is contained in the report.
So, in my defense; there is no evidence that connects the two reports to the stone initially presented to this forum.
Respectfully, I am in no way saying you are wrong, or that the lab reports are wrong. The evidence is just not consistent.
Do you have images of the stone contained in the LFG report? Not the image in the report, but images of the sample before the LFG analysis. Secondly, the SEM report (according to the dialog you posted) uses a sample encased in resin to stabilize a thin film strata coupon, typically used in a SEM analysis. This sample should have been extracted from the sample provided for the LFG report. Are there images of that?
This would be the evidence necessary to provide continuity.
Without that data, we are back to square one; my visual analysis.